The Relationship Between Concentration And Absorbance
The amount absorbed determines which color you see when you look at the solution. There is a relationship between concentration and absorbance.
Chem 125 Experiment Ii
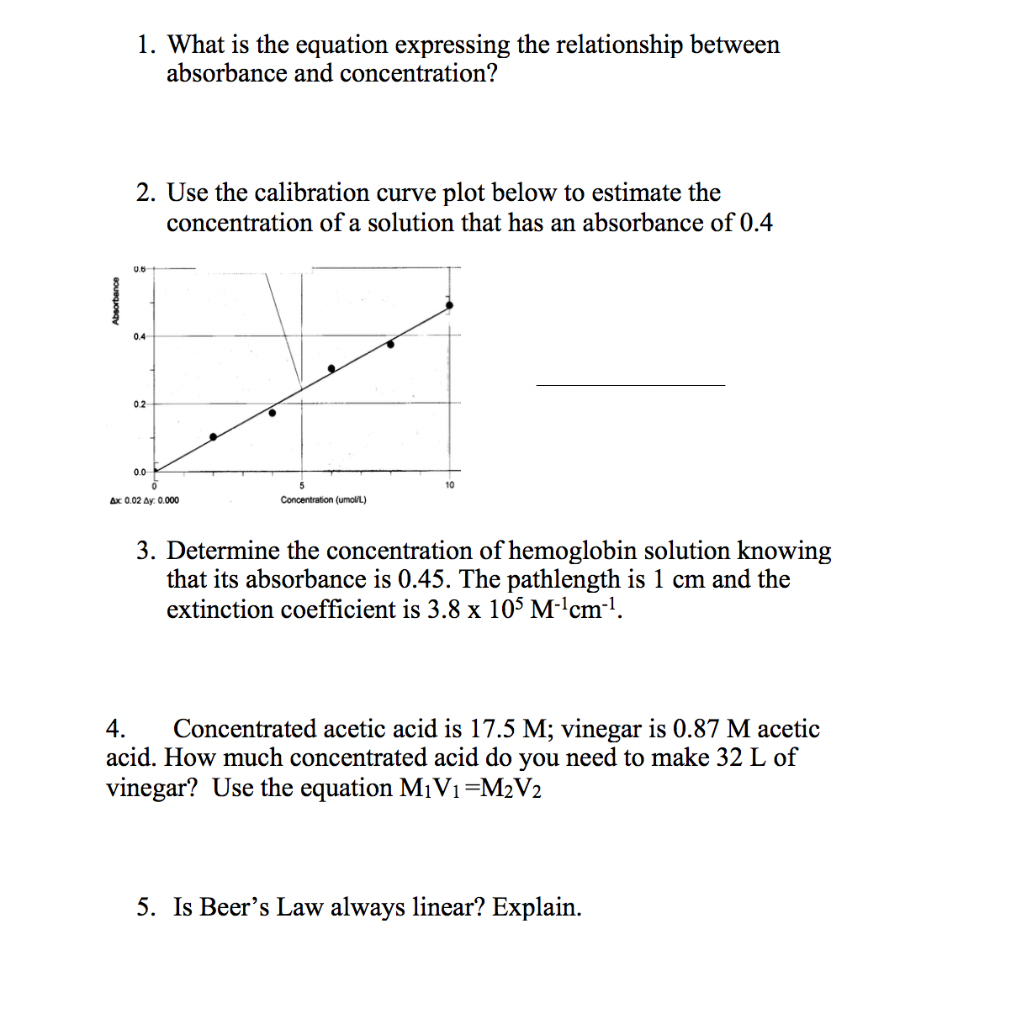
Solved 1 What Is The Equation Expressing The Relationship Chegg Com
Solved Question 6 Status Tries Remaining 2 Points Chegg Com
There is a direct relationship between absorbance and concentration is the higher the absorbance of a substance the more concentrated its solution will be in water or another medium.
The relationship between concentration and absorbance. Absorbance to transmittance can also be determined using th3 calculator above. It is the linear relationship between absorbance and concentration of an absorbing species. Beers law describes the dependence of a proteins absorbance on its absorptivity coefficient its concentration and the pathlength of the incident light.
The standard equation for absorbance is A ɛ x l x c where A is the amount of light absorbed by the sample for a given wavelength ɛ is the molar absorptivity l is the distance that the light travels through the solution and c is the concentration of the absorbing species per unit volume. When a beam of light passes through a solution with color it absorbs some of that light. Where a is the Absorbance e is the Molar absorption coefficient l is the Path Length and c is the Concentration.
Concentration we are able to prepare a graph that can be used to determine concentrations of unknowns. This relationship is expressed by the Lambert-Beer law which is more commonly known as Beers law. If however the series represents independent measurements of a variable to show a trend eg.
The relationship between molar extinction coefficient ε. In other words the more material in the solution absorbs the light the less light will get through. A a b c where A is the measured absorbance a is a wavelength-dependent absorptivity coefficient b is the path length and c is the analyte concentration.
A catalyst is a substance that speeds up a chemical reaction but is not consumed by the reaction. Concentration of the absorbing compound in solution. This relationship is expressed by Beers Law Equation 1.
It is also referred to as optical density Absorbance is calculated as a logarithmic function of T. At last the quantitative relationship model between the sea ice area and the mean near-surface temperature derived by MODIS data in Liaodong Bay was established. When you take an absorbanc spectrum the molar absorptivity constant is changing up and down and all around but when you are working with a calibration plot and are only changing the concentration it shows a linear relationship between absorbance and concentration.
Understand the Beer-Lambert law for absorbance A ɛ x l x c. The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution which enables the concentration of a solution to be calculated by measuring its. Finally a point is reached beyond which there is only small increase in the rate of the reaction with increasing substrate concentration.
Figure 4 is Beers Law curve for the absorbance of an. At higher substrate concentration the rate of reaction increase smaller and smaller amount in response to increase in substrate concentration. The general Beer-Lambert law is usually written as.
Solute concentration then the trend or relationship can be modeled by calculating the best-fit line or curve by regression analysis see A Painless Guide to Statistics Do not connect the dots when the measurements. Percent is as follows. Main Difference Absorbance vs.
Through research we found that the mean near-surface temperature was the most important factor for affecting the formation and melt of sea ice in Liaodong Bay. Traditionally such an instrument is used to determine the relationship between a wavelength and color. The concentration is simply the moles L-1 M of the sample dissolved in the solution and the length is the length of the cuvette used for the absorbance measurement and is typically 1 cm.
Absorbance A is the flip-side of transmittance and states how much of the light the sample absorbed. J Plot a graph of time against concentration of MnO 4- ions. A log10 1T log10 IoI.
Calculate Absorbance using Beers Law. Absorbance and transmittance are two related but different quantities used in spectrometry. The relationship between activity and concentration is affected by many factors such as temperature pH etc.
ε is the extinction coefficient also referred to as the. A ε l c Equation 1 A is the absorbance observed. Measure the absorbance of the mixture in the cuvette every 20 seconds until the absorbance drops to 001.
The Beer-Lambert law or Beers law is the linear relationship between absorbance and concentration of an absorbing species. The linear relationship between absorbance and concentration displays that absorbance depends on the concentration. The main difference between absorbance and transmittance is that absorbance measures how much of an incident light is absorbed when it travels in a material while transmittance measures how much of the light is transmitted.
This plateau is called maximum velocity V max. Percent molecular weight of protein Still other sources provide protein absorbance values for 01 mgmL solutions as this unit of measure is more. A standard curve of optical density vs.
This law states that the absorbance of a light absorbing material is proportional to its concentration in solution. 1 Note that although the samples tested to define LoB are devoid of analyte a blank zero sample can produce an analytical signal that might otherwise be consistent with a low concentration of analyte. Molar 10 ε.
Beers Law ONLY is linear at LOW concentrations. EP17 defines LoB as the highest apparent analyte concentration expected to be found when replicates of a sample containing no analyte are tested. According to this relationship the concentration of the analyte can be calculated when ε is known L is fixed and I 0 and I are.
10 concentration in mgml. A graph of absorbance vs concentration is called a Beers Law curve in honor of the chemist who first discovered the relationship between absorbance and concentration. In chemistry this is a principle known as the Beer-Lambert Law.
Mean price of computer memory over time. Molar and percent extinction coefficient ε. What is meant catalyst.
Normally ε at the wavelength of maximum absorption λ max is employed in quantitative analysis as errors resulting from instrumental wavelength uncertainty are minimized at the peak of the absorbance curve. Relationship has been exploited for the spectrophotometric determination of protein concentrations and it is defined by the BeerLambert law or Beers law. Beers Law AEbc helped to develop the linear equation since absorbance was equal to y Eb was equal to m and the concentration c was equal to the slope x in the equation ymxb.
Hence a catalyst can be recovered chemically unchanged at the end of the reaction it has been used to speed up or catalyze. While working in concentration units of molarity the Beers law is written as aecl. I Using the calibration curve convert the absorbance values obtained into concentrations of MnO 4-.

Spectrophotometer Lab

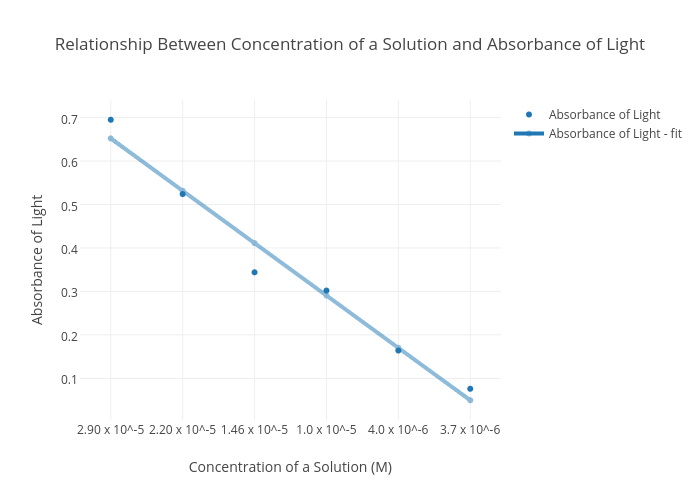
Relationship Between Concentration Of A Solution And Absorbance Of Light Scatter Chart Made By 161ciambronea
Untitled Document

1 2 Beer S Law Chemistry Libretexts
/beers-law-definition-and-equation-608172_FINAL-20ddc4fef437472db0a0ebe395770c76.png)
Beer S Law Definition And Equation
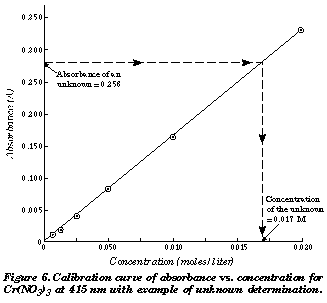
How Do You Calculate Concentration From Absorbance Socratic

Qms Week 5 Flashcards Quizlet
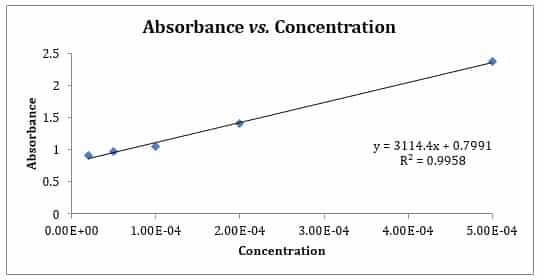
Beer S Law Lab Explained Absorbance Vs Concentration Schoolworkhelper
